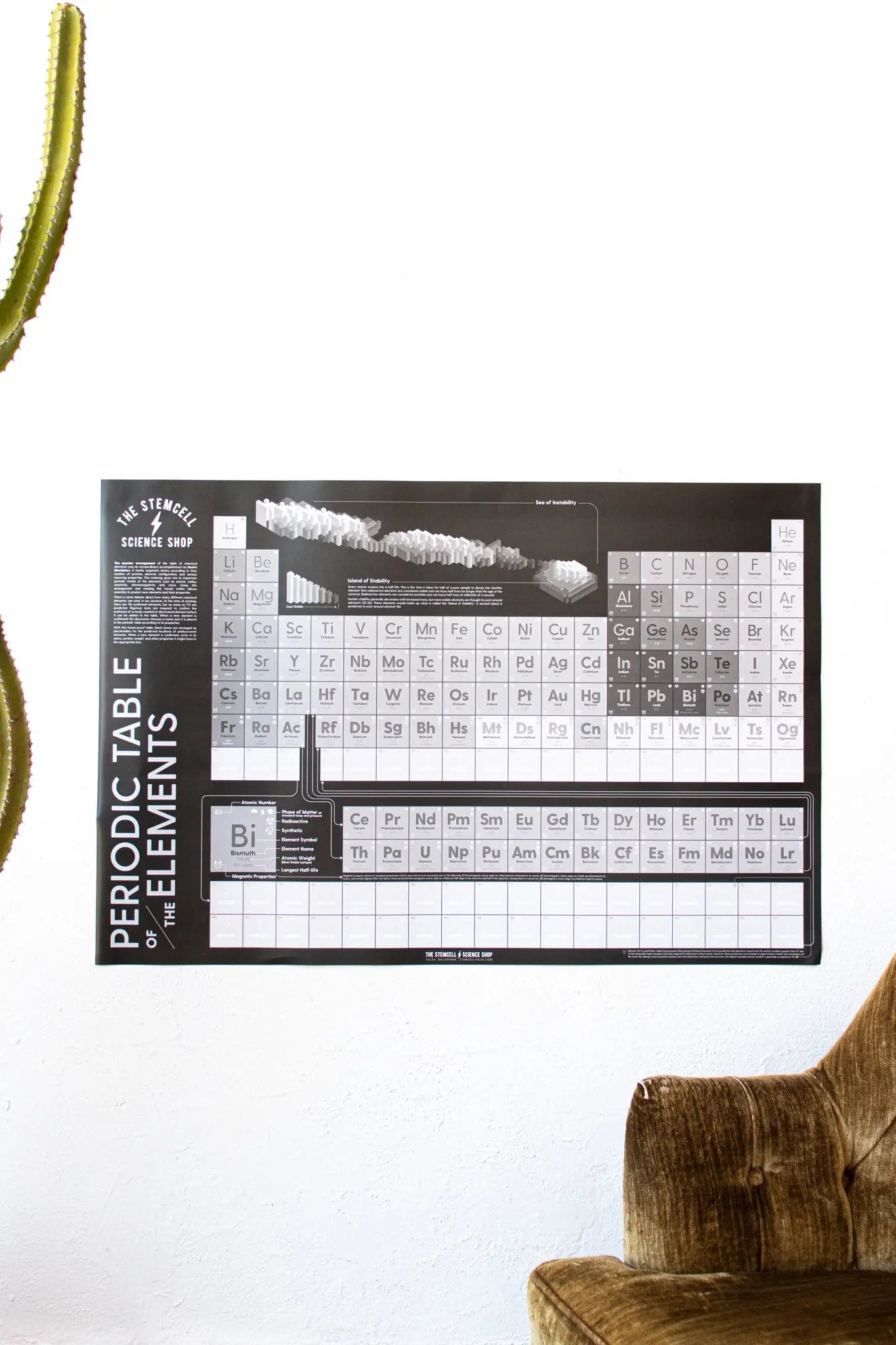
Future-Proof Periodic Table of the Elements
Future-Proof Periodic Table of the Elements
5.0 / 5.0
(3) 3 total reviews
Introducing the last table of elements you'll ever need buy.
Existing tables become outdated every time a new element is discovered, and labs/scientists/classrooms around the world must buy updated tables. We thought this was ridiculous, so we made ours future-proof.
By adding blank boxes as placeholders for the predicted, undiscovered elements (according to the patterns created by the known elements), our table will never become obsolete.
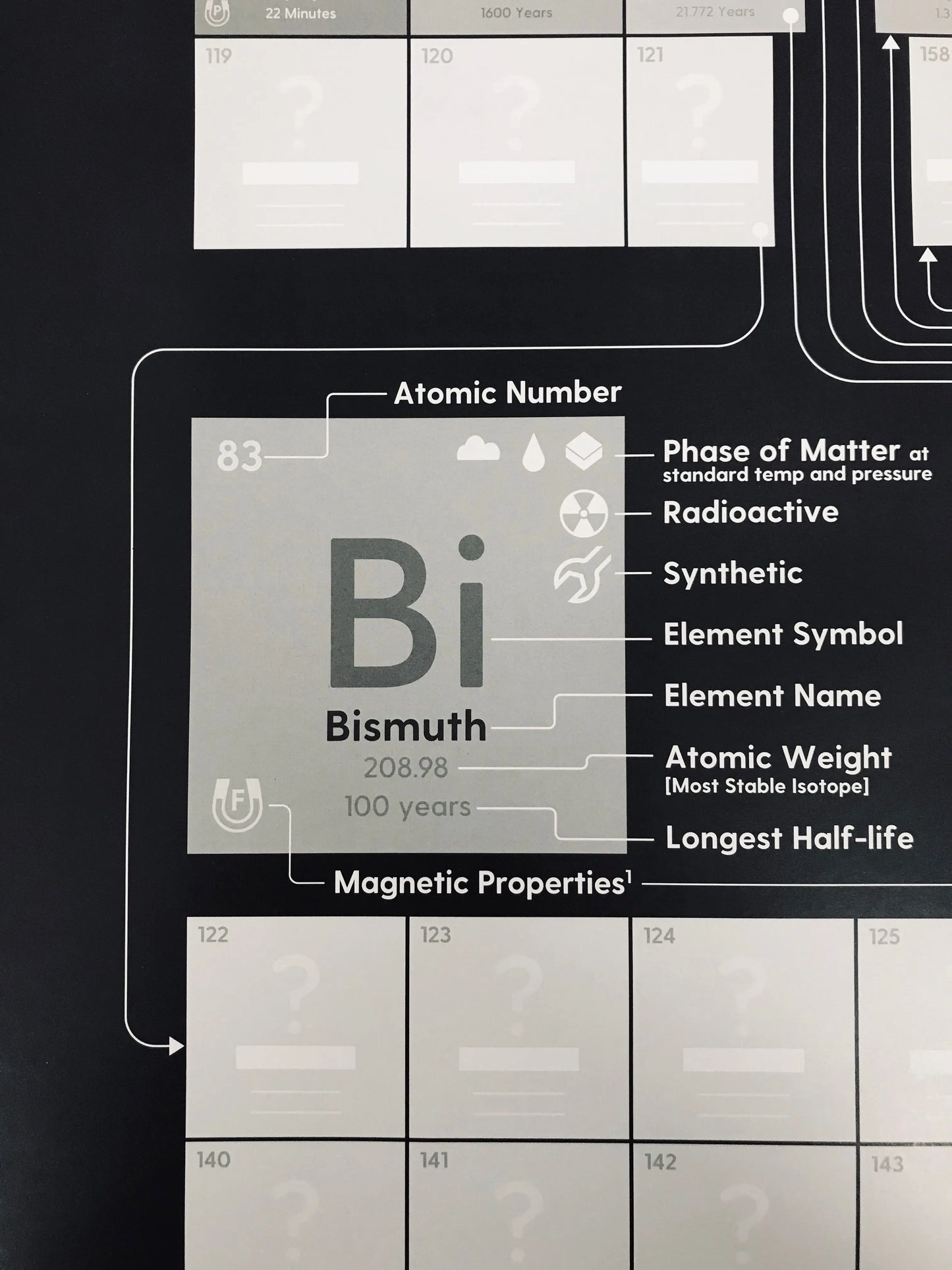
When a new element is confirmed, write in its name, symbol, weight, and other properties in the appropriate box.
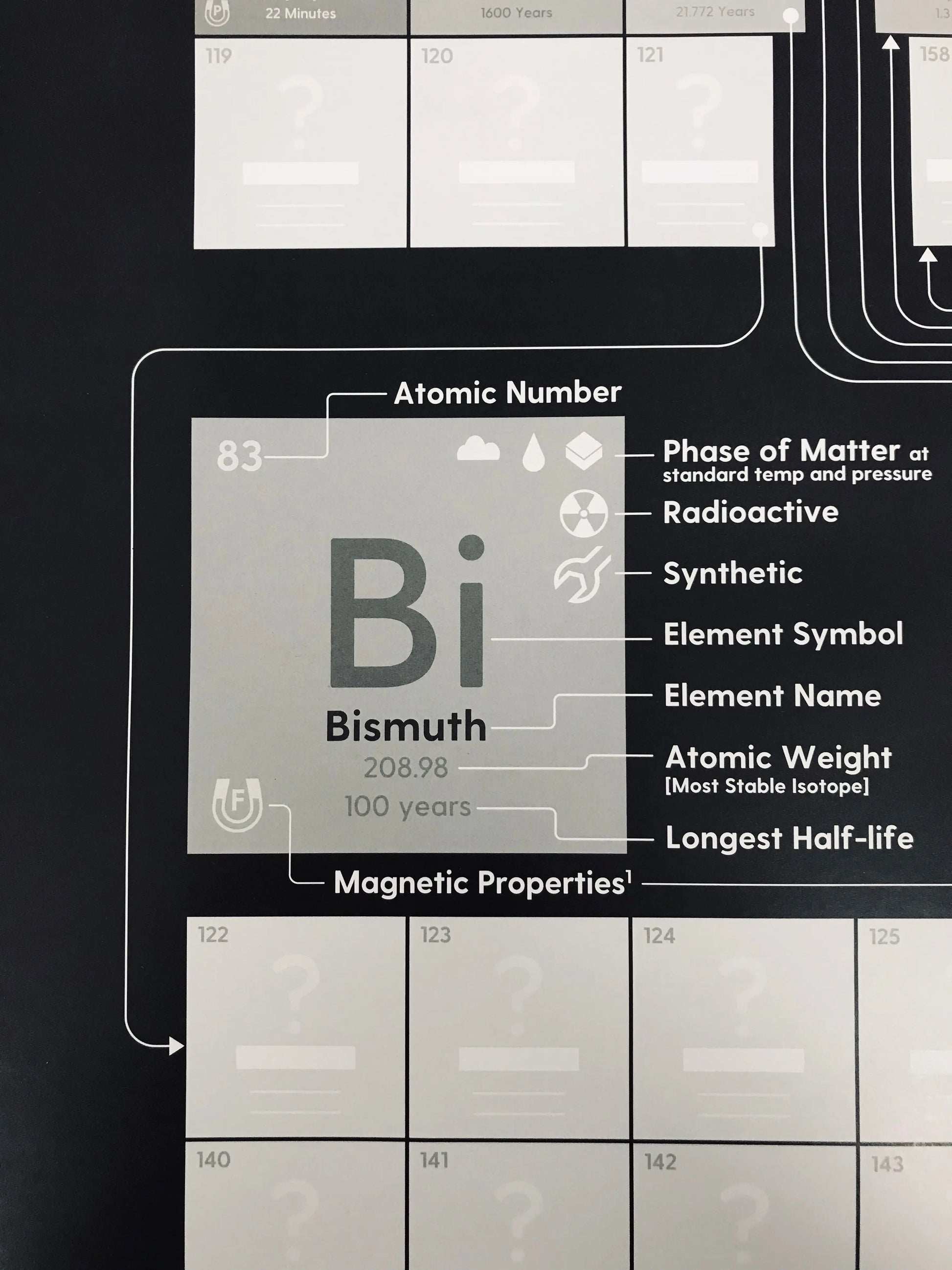
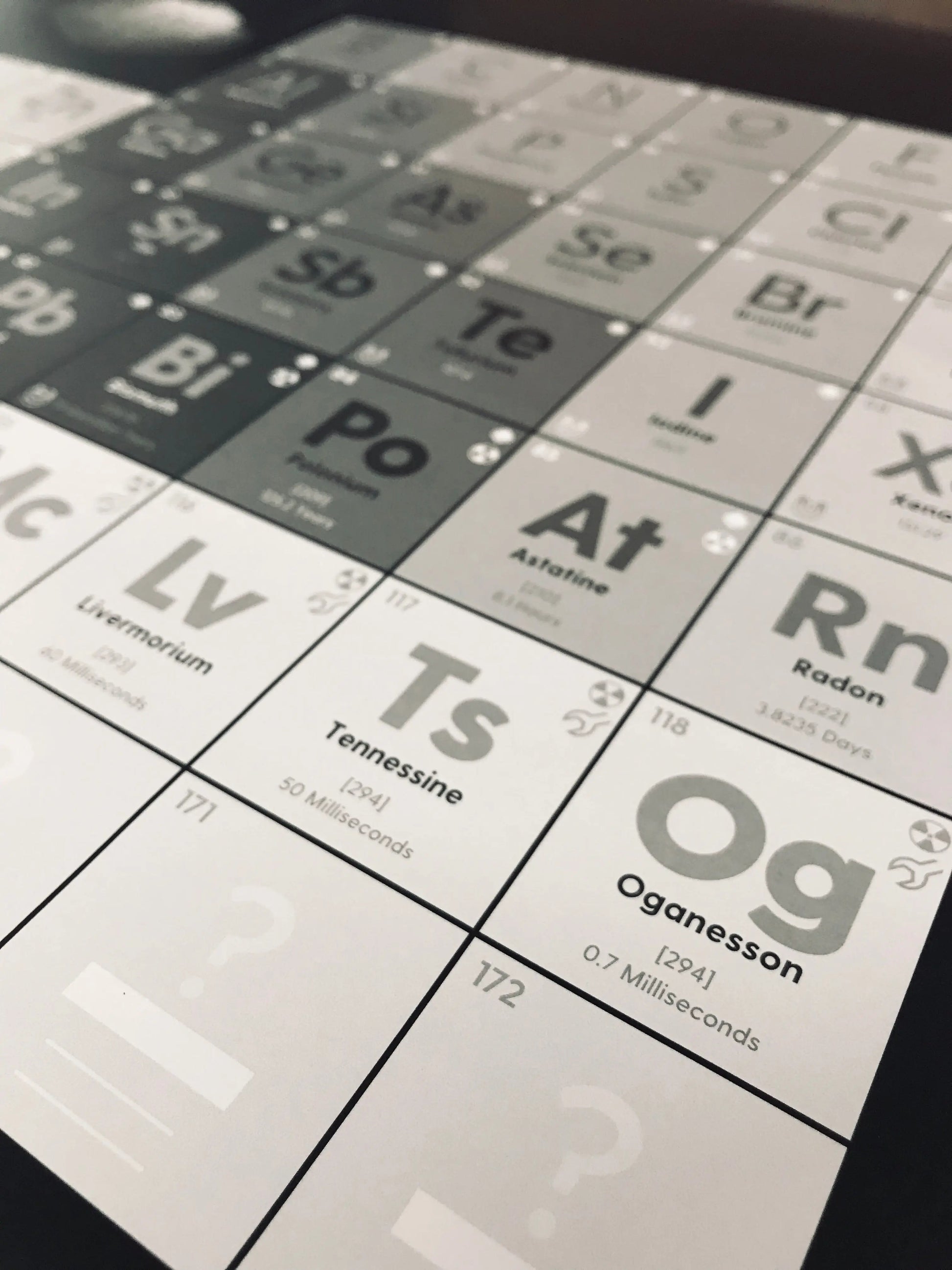
We've included the following info about the 118 known elements:
✅ Atomic weight (also indicated by the font weight)
✅ Font weight is also indicative of atomic weight
✅ Phase of matter at standard temperature & pressure
✅ Magnetic properties
✅ Synthetic or naturally-occurring
✅ Radioactivity indicator
✅ The longest half-life of radioactive elements
Additional features:
✅ Feynamium indicator
✅ Island of Stability information
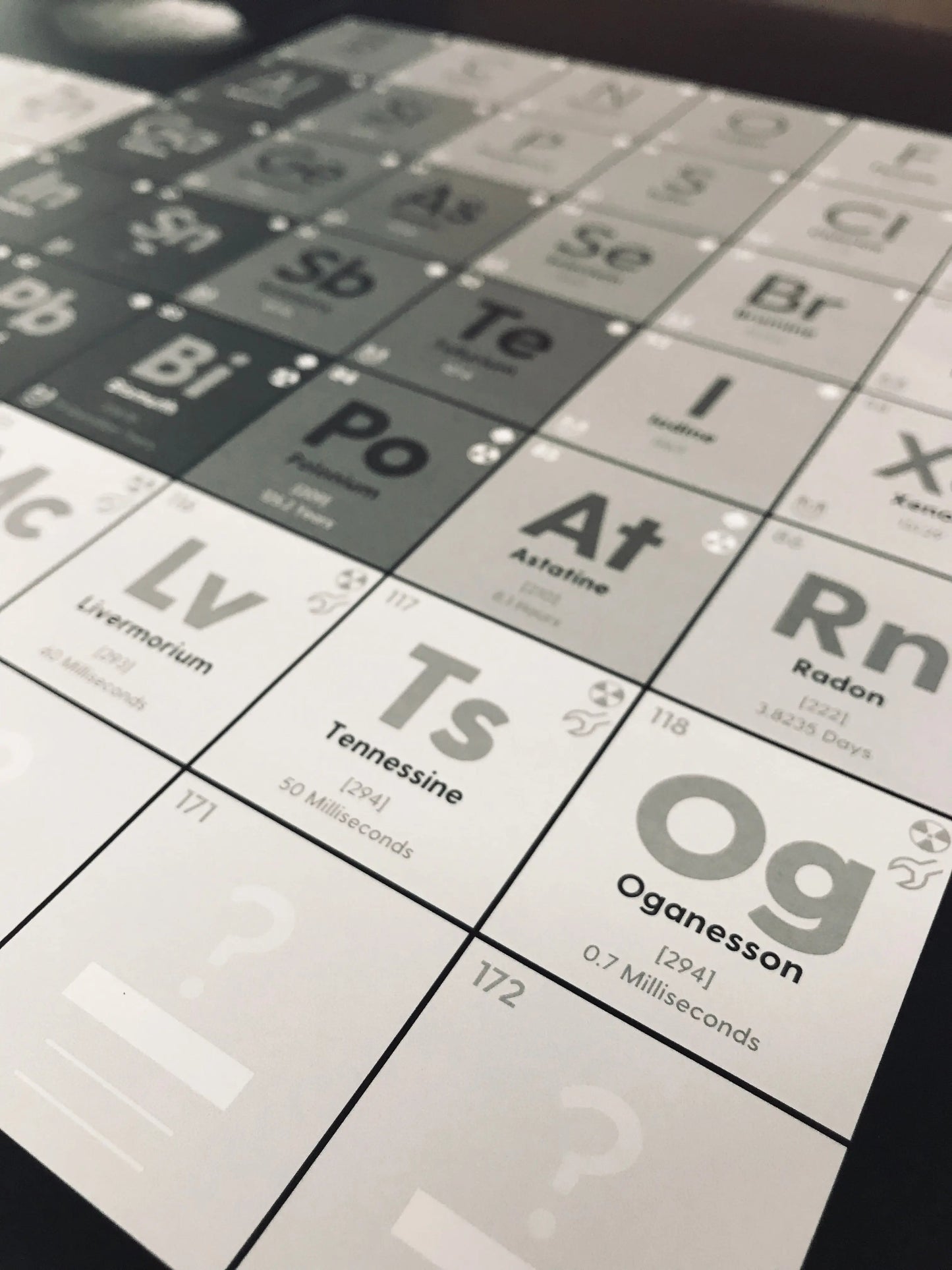
✅ 172 elements (the placement of element 173 is unknown)
✅ Dimensions: 24" x 36"
✅ Neutral color-scheme to complement any room.
More info:
The popular arrangement of the table of chemical elements was an extraordinary accomplishment by Dmitri Mendeleev. It neatly organizes atoms according to their number of protons, electron configuration, and various recurring properties. This ordering gives rise to important periodic trends of the elements such as atomic radius, reactivity, electronegativity and more. Using this arrangement and reading the trends within allows scientists to predict new elements and their properties.
There is some debate about how many different chemical elements can exist in our universe. At the time of printing, there are 118 confirmed elements, but as many as 173 are predicted. Rigorous tests are required to confirm the existence of a newly created or discovered element before it can be added to the table. When a new element is confirmed, the discoverer chooses a name and it is placed in the periodic table according to its properties.
Matter Subscriber Price:
Couldn't load pickup availability



Collections containing this item:
Bestsellers
|
Charts
|
Chemistry
|
Element Samples
|
Household
|
Items ≤ $20
|
Newest Listings
|
Stemcell Catalog
|
Wholesale Products
|
AUTHENTICITY GUARANTEED
We only list 100% verified authentic items. We work with reputable collectors, and regularly consult with our network of scientists and experts.
-
Free Shipping
Orders ≥$50 qualify for free US shipping
Orders ≥$100 qualify for free global shipping -
Secure Payments
Pay how you like: credit card, PayPal, After Pay, Shop Pay, Venmo, Apple/Google/Meta Pay & crypto
-
Simple Returns
Change your mind? No problem. Enjoy easy returns within 30 days.
Super fantastic bubble plastic. Highly recommend
Looks great!
Love ❤️
similar items_
WE TAKE SCIENCE SERIOUSLY
You deserve better than craft store science products. And we think learning is more impactful when you're holding a tangible piece of what you're learning about. That's why Stemcell exists.
We're dedicated to providing the best scientific products available—whether they're fragments of scientific importance, experimental activities, or just interesting things that scratch your curiosity itch.
With every new product launch, our list of new ideas gets longer rather than shorter. So check in often for our latest projects, and thanks for being a part of our endeavor to make the world a smarter place to live.
Believe in yourself; for everything else, there's science.
— TERRY MUDGE, FOUNDER & SCIENTIFIC DIRECTOR